ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Last updated 02 junho 2024

Baseline predictors of (A) ASDAS ID and (B) ASAS PR at week 12 of

AS Criteria: Diagnosis (ASAS), Disease Activity (ASDAS), Radiographic Progression (mSASSS) - Arthritis Rheumatism
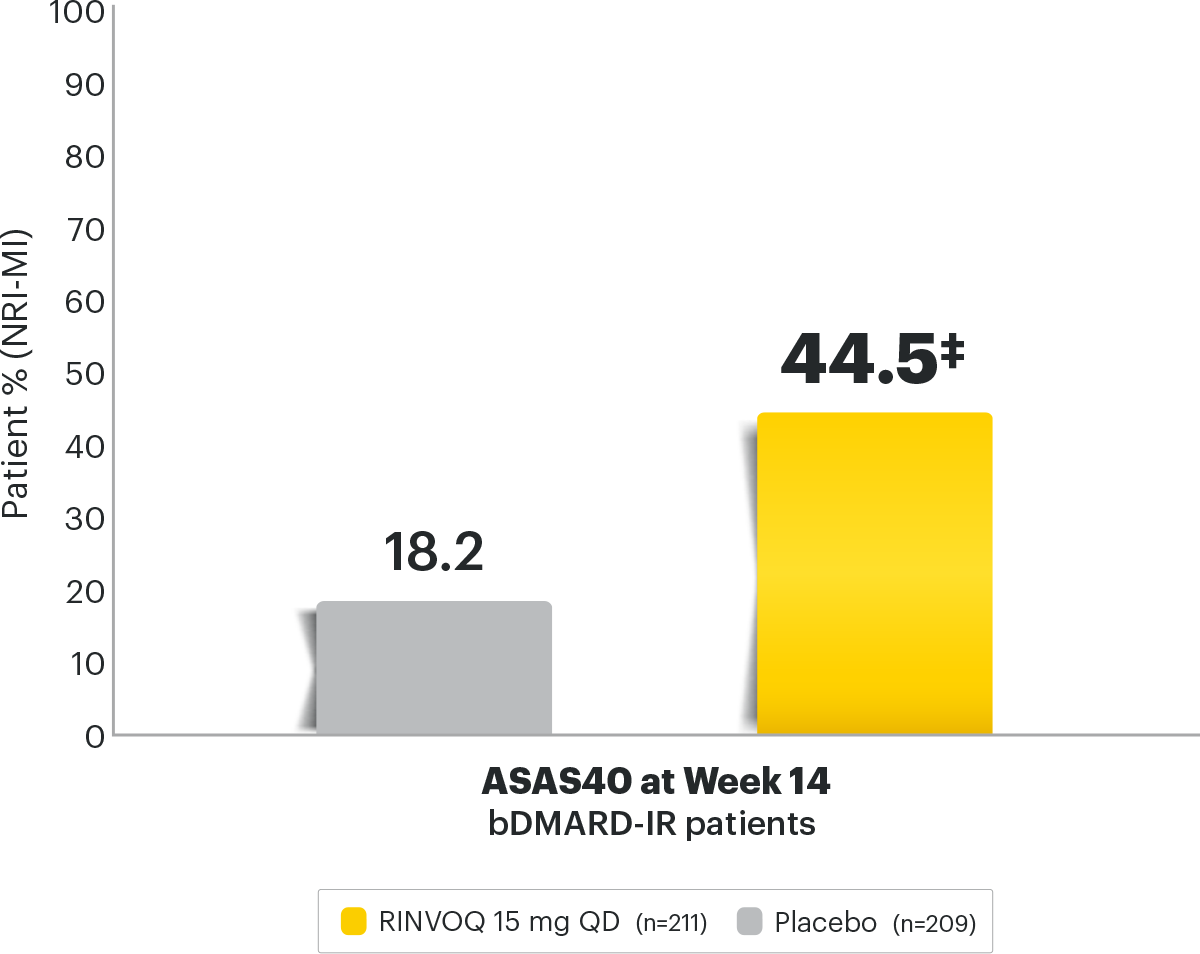
Axial Spondyloarthritis RINVOQ® (upadacitinib)

Disease Control Data, Ankylosing Spondylitis

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis

Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. A systematic review - ScienceDirect
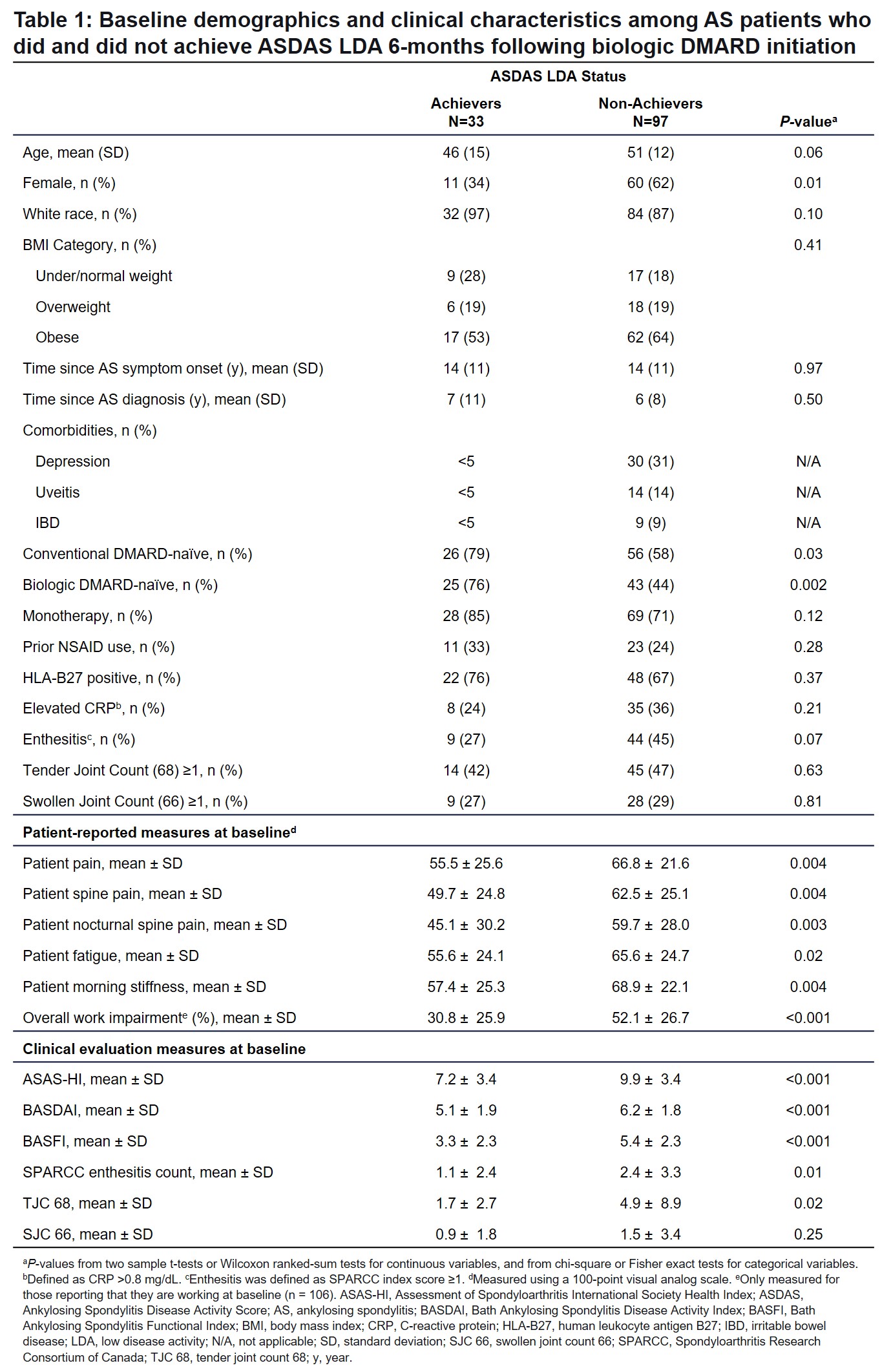
Impact of Achieving ASDAS LDA on Disease Activity and Patient-Reported Outcome Measures Among Patients with Ankylosing Spondylitis Treated with Biologic DMARDs - ACR Meeting Abstracts

Safety and Efficacy of Upadacitinib in Patients With Active Ankylosing Spondylitis and an Inadequate Response to Nonsteroidal Antiinflammatory Drug Therapy: One‐Year Results of a Double‐Blind, Placebo‐Controlled Study and Open‐Label Extension - Deodhar

Disease Control Data, Ankylosing Spondylitis
Recomendado para você
-
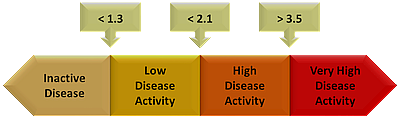 ASDAS Calculator02 junho 2024
ASDAS Calculator02 junho 2024 -
ASAS_APP Did you know that ASAS has an app to facilitate the calculation of ASDAS? – click on this link to find out - ASAS - Assessment of SpondyloArthritis international Society02 junho 2024
-
 ASDAS states in patients stratified by baseline MRI/CRP status.02 junho 2024
ASDAS states in patients stratified by baseline MRI/CRP status.02 junho 2024 -
 Asda sales growth accelerates in latest quarter02 junho 2024
Asda sales growth accelerates in latest quarter02 junho 2024 -
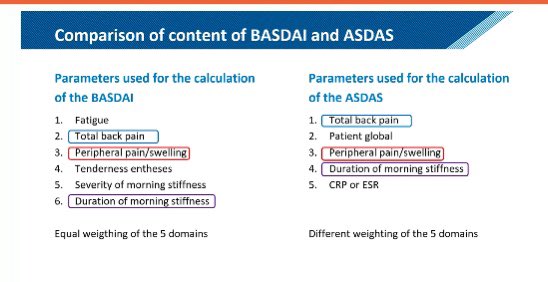 SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X02 junho 2024
SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X02 junho 2024 -
 Mean Ankylosing Spondylitis Disease Activity Score with C‐reactive02 junho 2024
Mean Ankylosing Spondylitis Disease Activity Score with C‐reactive02 junho 2024 -
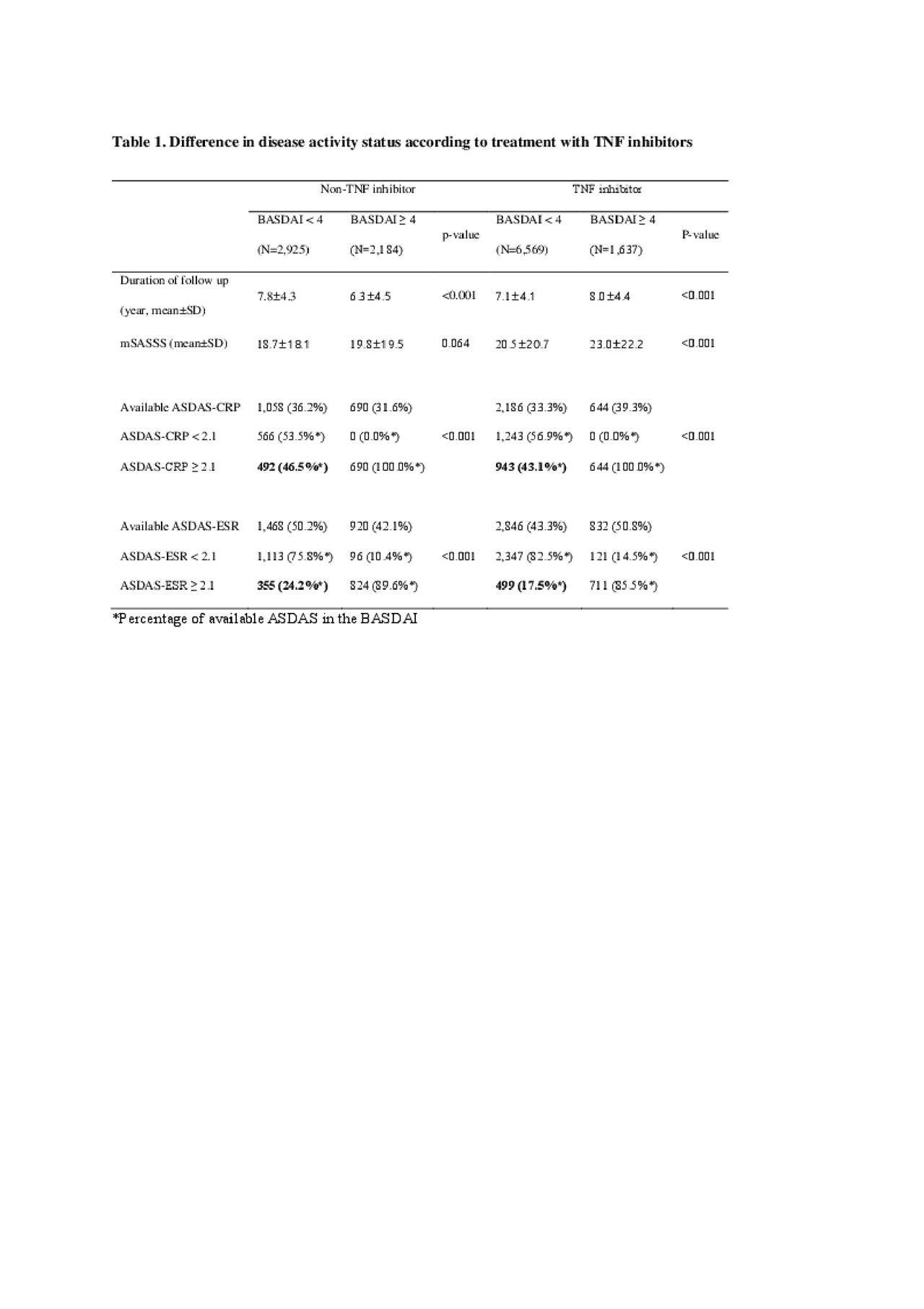 ASDAS Is More Important Than BASDAI in Advanced Ankylosing Spondylitis - ACR Meeting Abstracts02 junho 2024
ASDAS Is More Important Than BASDAI in Advanced Ankylosing Spondylitis - ACR Meeting Abstracts02 junho 2024 -
 Asda delivery drivers use in-cab technology to cut CO202 junho 2024
Asda delivery drivers use in-cab technology to cut CO202 junho 2024 -
 Adobe Audition CC icon with random file name asdas.sesx | Art Print02 junho 2024
Adobe Audition CC icon with random file name asdas.sesx | Art Print02 junho 2024 -
 Asda expands on Uber Eats - New Food Magazine02 junho 2024
Asda expands on Uber Eats - New Food Magazine02 junho 2024
você pode gostar
-
 BRINQUEDO O Show da Luna Boneca de coleção em02 junho 2024
BRINQUEDO O Show da Luna Boneca de coleção em02 junho 2024 -
 Curso online grátis: 58 sites que oferecem cursos online e gratuitos02 junho 2024
Curso online grátis: 58 sites que oferecem cursos online e gratuitos02 junho 2024 -
 Code of War MOD APK 3.18.7 (Unlocked VIP) for Android02 junho 2024
Code of War MOD APK 3.18.7 (Unlocked VIP) for Android02 junho 2024 -
Shawty Like a Melody (Ambient Remix) by nEWfOLDERxlsx (Single, Ambient): Reviews, Ratings, Credits, Song list - Rate Your Music02 junho 2024
-
 Call Of Duty Vanguard Zombies Gameplay Reveal Teasers Maps, Perks, Operators & Treyarch Plot Twist02 junho 2024
Call Of Duty Vanguard Zombies Gameplay Reveal Teasers Maps, Perks, Operators & Treyarch Plot Twist02 junho 2024 -
 EA FC 24 Companion App Release Time02 junho 2024
EA FC 24 Companion App Release Time02 junho 2024 -
 kaguya sama: love is war - O Vício02 junho 2024
kaguya sama: love is war - O Vício02 junho 2024 -
 A Studio Break-In Cost The World A Live-Action King Of The Hill02 junho 2024
A Studio Break-In Cost The World A Live-Action King Of The Hill02 junho 2024 -
 Hot Wheels Monster Trucks, Oversized Monster Truck Bone Shaker, 1:24 Scale Die-Cast Toy Truck with Giant Wheels and Cool Designs : Toys & Games02 junho 2024
Hot Wheels Monster Trucks, Oversized Monster Truck Bone Shaker, 1:24 Scale Die-Cast Toy Truck with Giant Wheels and Cool Designs : Toys & Games02 junho 2024 -
 bendy and the ink machine, Tumblr02 junho 2024
bendy and the ink machine, Tumblr02 junho 2024
