What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 16 junho 2024

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

Computer System Validation (CSV) in Life Sciences Part 1: Introduction to CSV - Verista
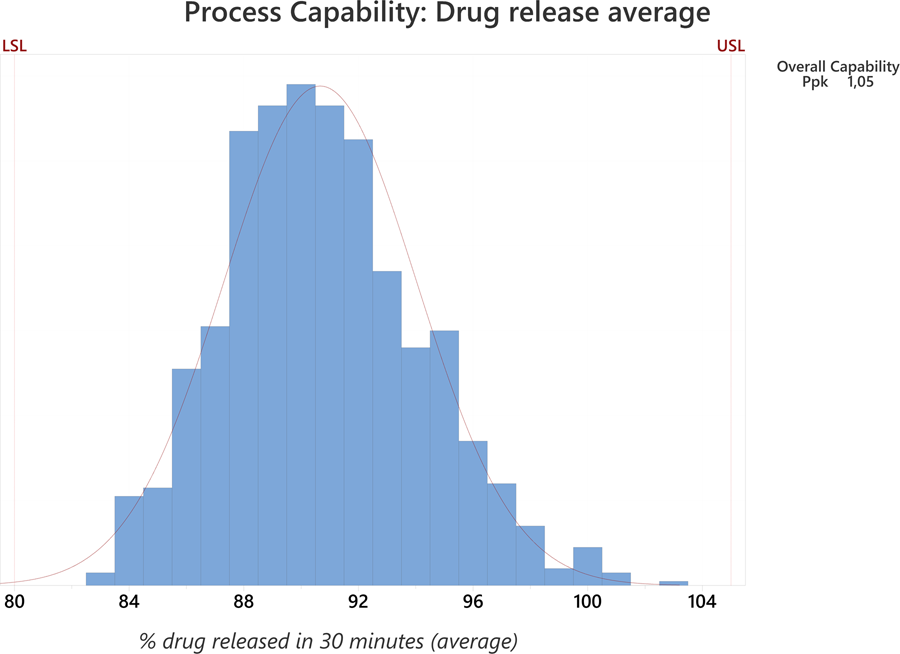
Big data collection in pharmaceutical manufacturing and its use for product quality predictions
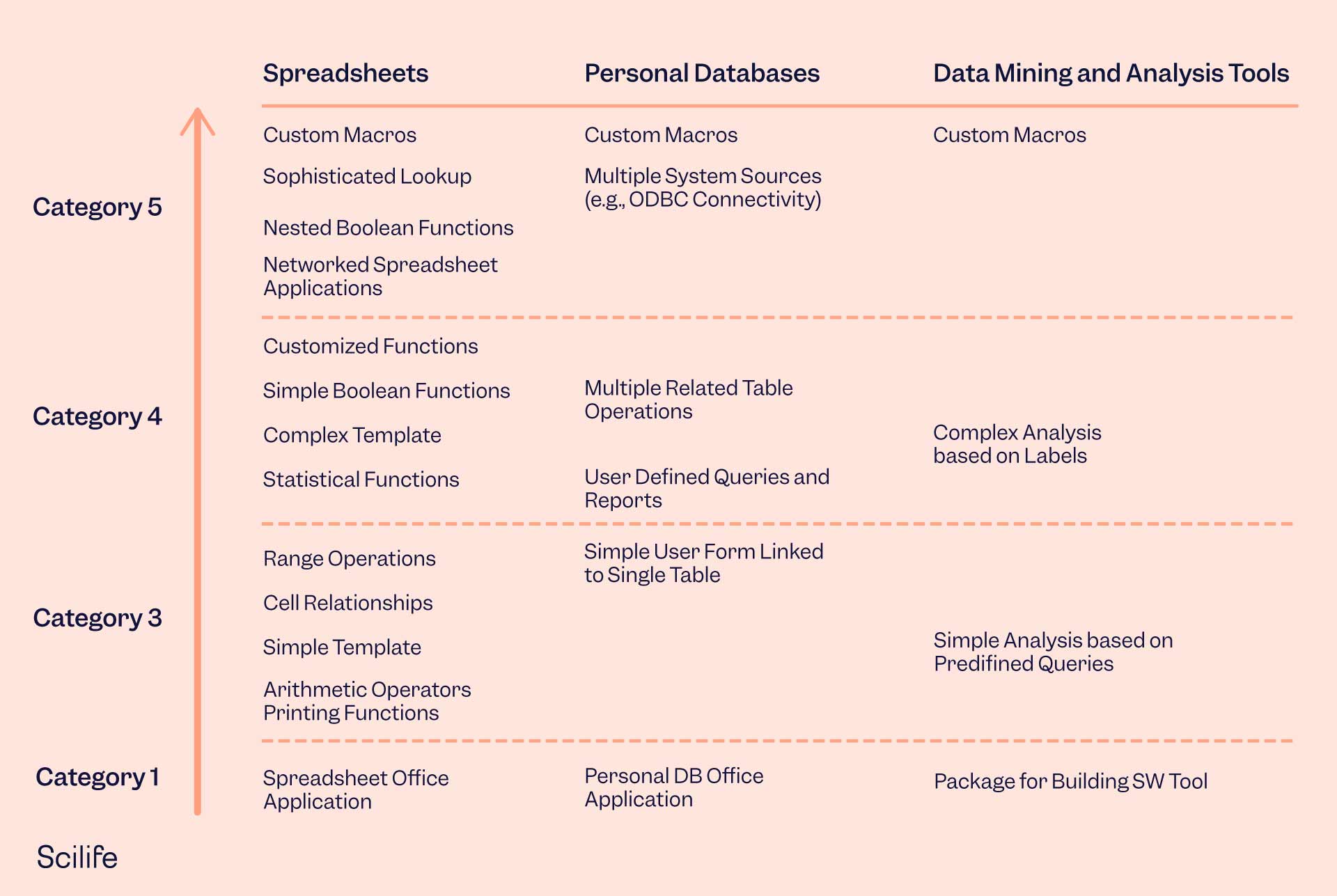
CSV vs. CSA: What Are the Main Differences?

Data Integrity in the Pharmaceutical Industry

Computer System Validation
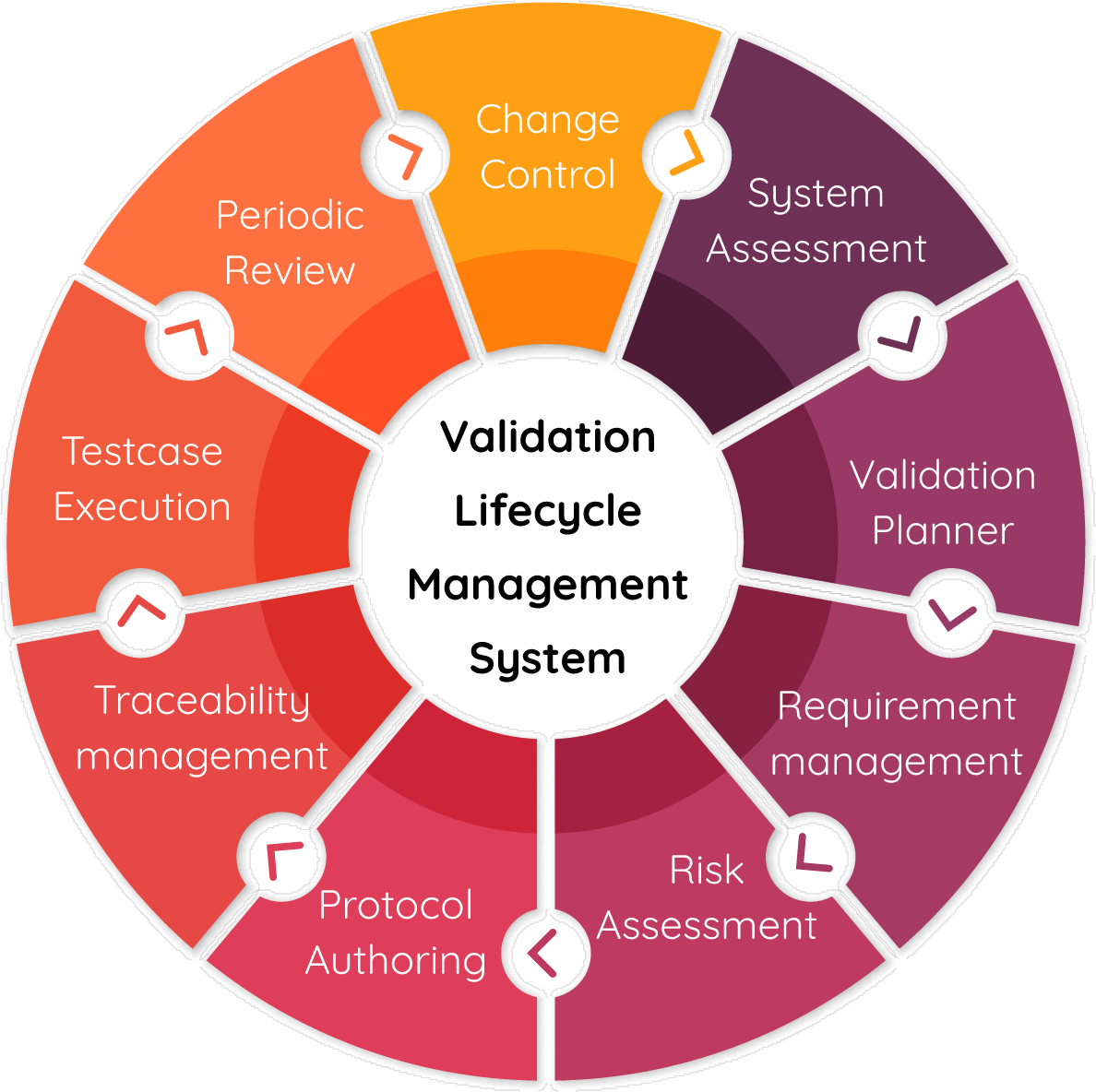
Computer System Validation (CSV) Services, Clinical Services, Services

9 of the Best Online CSV Resources for Pharma Validation Professionals

Complete guide to computer system validation in 2023
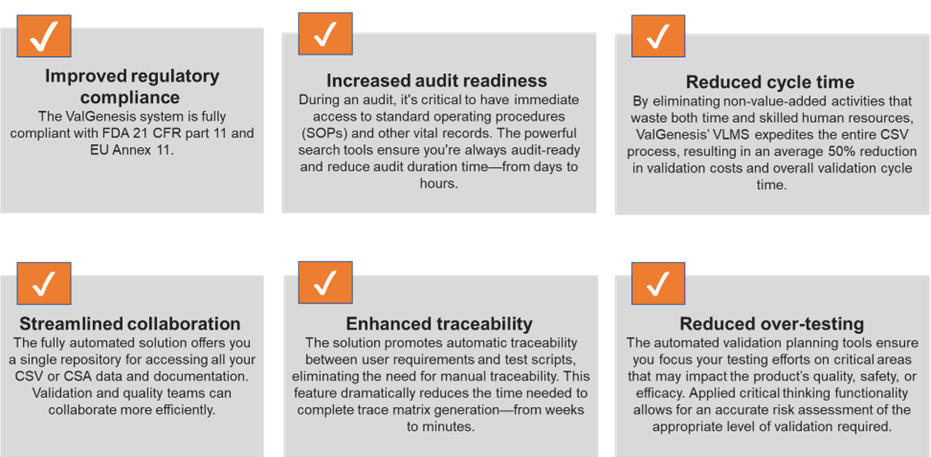
6 Common Challenges of Paper-Based computer system validation (CSV) and How to Digitize Them - Astrix
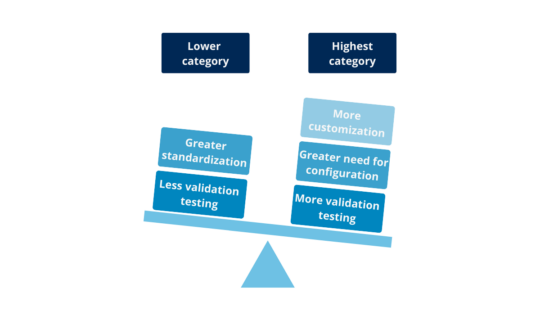
A Complete Guide to Computer System Validation (CSV): What is it and why do we need it?
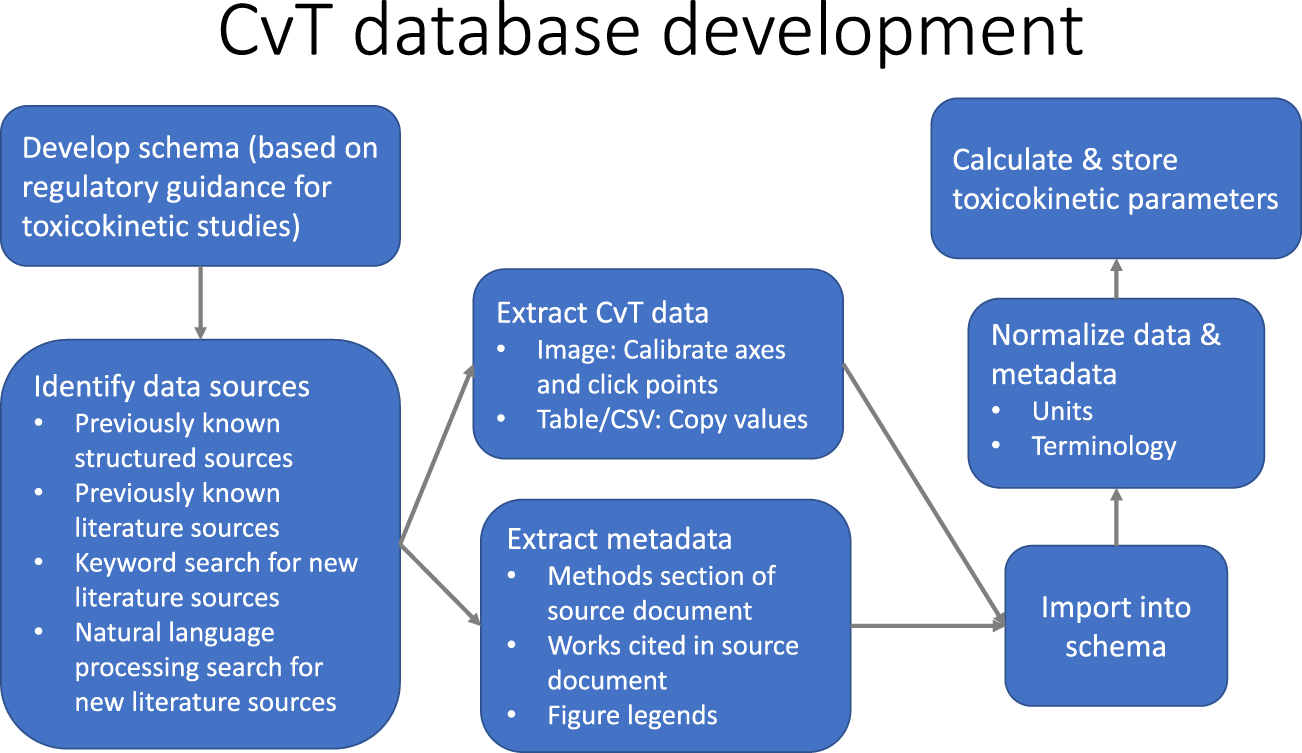
Database of pharmacokinetic time-series data and parameters for 144 environmental chemicals
CSV is a Profession Too

Software Validation / Computer System Validation (CSV) /

Why is Computer System Validation so important? - Express Pharma

Types of Validation in the Pharmaceutical Industry
Recomendado para você
-
 School São Vicente de Paulo - São Luís16 junho 2024
School São Vicente de Paulo - São Luís16 junho 2024 -
Colégio São Vicente de Paulo16 junho 2024
-
Escola Deuzuita de Queiroz vai participar de Feira de Tecnologia16 junho 2024
-
 Addgene: pAAV-CAG-SomaGCaMP6f216 junho 2024
Addgene: pAAV-CAG-SomaGCaMP6f216 junho 2024 -
 Pacific Merchant Shipping Association16 junho 2024
Pacific Merchant Shipping Association16 junho 2024 -
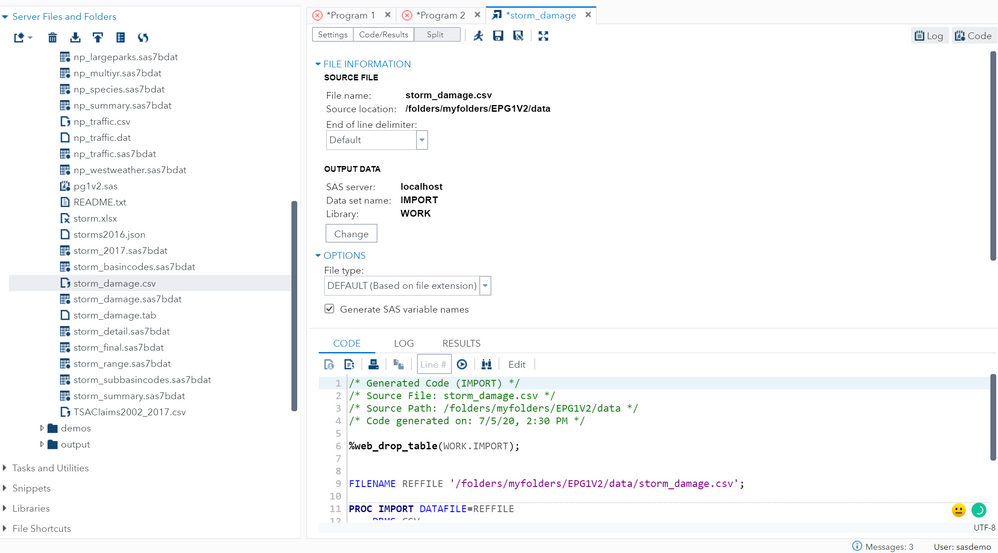
import csv file - SAS Support Communities16 junho 2024
-
 Afiliados - SINEPE/MA16 junho 2024
Afiliados - SINEPE/MA16 junho 2024 -
 Managing organizations via CSV Import — Zammad Admin Documentation16 junho 2024
Managing organizations via CSV Import — Zammad Admin Documentation16 junho 2024 -
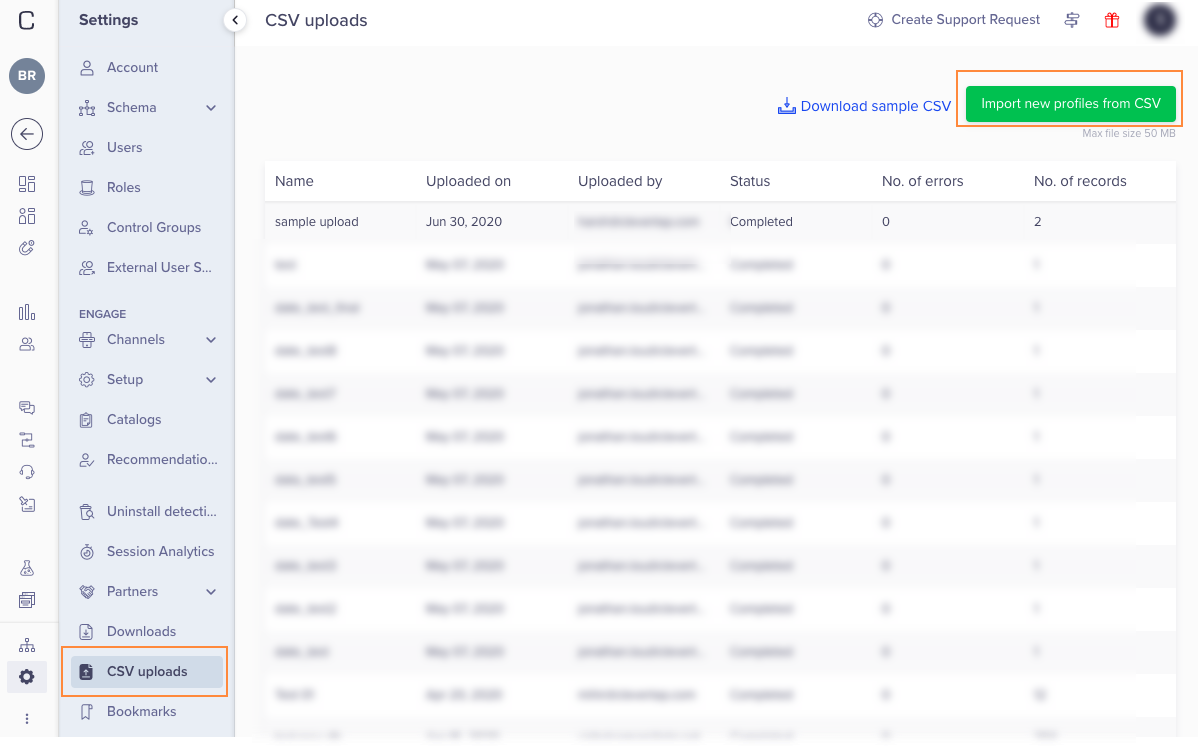 CSV Upload16 junho 2024
CSV Upload16 junho 2024 -
 2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars16 junho 2024
2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars16 junho 2024
você pode gostar
-
 minecraft novaskin wallpaper ဒေါင်းနည်း #harrymyanmar##myanmar#harry16 junho 2024
minecraft novaskin wallpaper ဒေါင်းနည်း #harrymyanmar##myanmar#harry16 junho 2024 -
 Perspectives on Crime: An Introduction to Criminal Justice: 9781524900434: Eric Ling, Pj Verrecchia: Books16 junho 2024
Perspectives on Crime: An Introduction to Criminal Justice: 9781524900434: Eric Ling, Pj Verrecchia: Books16 junho 2024 -
Tiara Orelha Gato (Grande) - Cosplay Fantasia Orelhas Neko Geek16 junho 2024
-
 dinamica de perguntas com criancas16 junho 2024
dinamica de perguntas com criancas16 junho 2024 -
 Charlie Murder - Wikipedia16 junho 2024
Charlie Murder - Wikipedia16 junho 2024 -
 Getafe CF - patrocinador16 junho 2024
Getafe CF - patrocinador16 junho 2024 -
 Roupas de boneca em crochê! Artesanal e perfeitas - Artigos16 junho 2024
Roupas de boneca em crochê! Artesanal e perfeitas - Artigos16 junho 2024 -
 Google Play Store: surgimento da loja de apps do Android16 junho 2024
Google Play Store: surgimento da loja de apps do Android16 junho 2024 -
 Yuno Gasai (Mirai Nikki / Future Diary) + Yandere Trance - Yuno16 junho 2024
Yuno Gasai (Mirai Nikki / Future Diary) + Yandere Trance - Yuno16 junho 2024 -
 Os Ausentes Conheça a primeira série brasileira original da HBO Max - Canaltech16 junho 2024
Os Ausentes Conheça a primeira série brasileira original da HBO Max - Canaltech16 junho 2024